Introduction
The Next-Generation Biomanufacturing Market refers to advanced bioproduction systems that use modern technologies such as continuous bioprocessing, synthetic biology, single-use systems, and AI-driven process controls to enhance the efficiency, scalability, and cost-effectiveness of biological product manufacturing. These solutions support the production of vaccines, monoclonal antibodies, cell and gene therapies, recombinant proteins, and other biologics.
Global importance is rising as healthcare systems increasingly depend on high-quality biologics for disease treatment, prevention, and personalized medicine. The need for faster production cycles, lower operational costs, improved product yields, and flexible manufacturing platforms drives demand for next-generation solutions. The growing burden of chronic and infectious diseases and the expansion of biopharma R&D make next-generation biomanufacturing a critical foundation for future medical advancements.
Learn how the Next-Generation Biomanufacturing Market is evolving—insights, trends, and opportunities await. Download report: https://www.databridgemarketresearch.com/reports/global-next-generation-biomanufacturing-market
The Evolution
Biomanufacturing has transitioned from traditional stainless-steel batch systems to flexible and automated processes. Early bioproduction relied heavily on large-scale fixed bioreactors, extensive cleaning cycles, and high capital expenditure. As the biologics market expanded, new methods emerged to address efficiency challenges.
Key innovations included single-use bioreactors, improved chromatography systems, and better cell-line engineering. Continuous biomanufacturing represented a major milestone, enabling uninterrupted processing with consistent product quality and reduced costs. Synthetic biology expanded design capabilities, enabling engineered cells with higher productivity.
Shifts in demand accelerated innovation. The rise of complex biologics such as CAR-T therapies created the need for personalized, small-batch production. The global pandemic highlighted the necessity for rapid vaccine manufacturing platforms. Technology advancements in automation, digital twins, machine learning, and real-time analytics changed how facilities are designed and operated.
Next-generation systems now focus on flexibility, modularity, sustainability, and digital integration. These developments position the industry to meet the rising global demand for innovative therapeutics.
Market Trends
Consumer and industry trends reflect a shift toward rapid, modular, and cost-efficient biomanufacturing infrastructure. Biopharma companies emphasize agility as product pipelines diversify and personalized medicine expands. Continuous manufacturing and integrated upstream-downstream processes reduce production timelines and minimize waste.
Technology adoption is accelerating in areas such as AI-assisted bioprocess design, predictive analytics, and smart sensors for quality monitoring. Single-use technologies and hybrid manufacturing platforms dominate new facility investments due to shorter installation times and lower contamination risks.
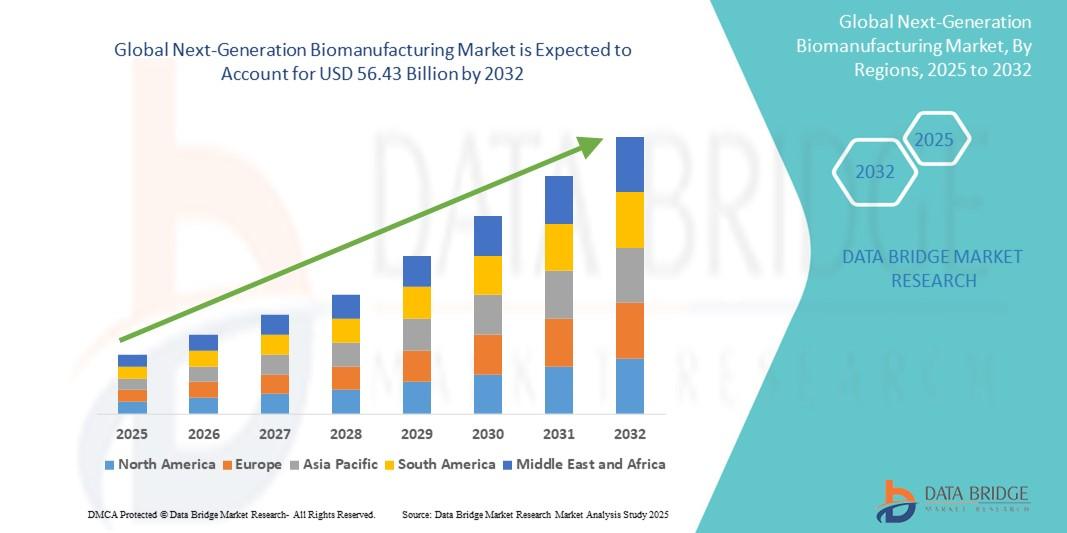
Regional and global trends show strong uptake in North America and Europe driven by established biologics industries and investments in advanced therapy manufacturing. Asia-Pacific demonstrates rapid expansion due to growing biotech clusters, manufacturing outsourcing, and government support for healthcare innovation.
Digitalization trends, sustainability goals, and the global shift toward decentralized manufacturing enable new facility models such as modular and portable bioprocessing units. These trends contribute to broader access to biologics worldwide.
Challenges
The Next-Generation Biomanufacturing Market faces several regulatory, economic, and operational challenges. Regulatory complexity arises from the dynamic nature of new therapies and production technologies. Approvals for advanced continuous systems require extensive validation and quality monitoring.
Economic challenges include high capital investment for advanced systems, operational expertise shortages, and rising costs of specialized raw materials. The global supply chain for bioprocessing equipment and consumables remains vulnerable to disruptions due to limited supplier diversity and high demand for essential components.
Key barriers to growth include the shortage of trained professionals capable of operating automated and AI-driven systems. Transitioning from legacy infrastructure requires significant retraining and facility redesign. Risks include data security concerns associated with digitalized bioprocessing, inconsistent raw material quality, and potential failures in automated control systems.
Ensuring consistent regulatory compliance during continuous production and scaling personalized therapies also remains a critical challenge for industry stakeholders.
Market Scope
Segmentation by Type
-
Continuous bioprocessing systems
-
Single-use bioreactors and components
-
Advanced cell culture systems
-
Process analytical technology (PAT) tools
-
Automated bioprocessing platforms
-
Modular and flexible manufacturing units
-
Synthetic biology-based production systems
Segmentation by Application
-
Vaccine manufacturing
-
Monoclonal antibody production
-
Recombinant protein development
-
Cell and gene therapy production
-
Biosimilar manufacturing
-
Microbial fermentation-based products
-
Personalized therapy manufacturing
Segmentation by Technology
-
Single-use technology
-
Continuous upstream processing
-
Continuous downstream processing
-
Smart bioreactor control systems
-
AI and machine-learning-based systems
-
Synthetic biology platforms
Regional Analysis
North America
Strong market presence driven by leading biotech companies, robust regulatory frameworks, and significant investment in cell and gene therapy manufacturing.
Europe
Large demand for biologics, strong academic-industry collaborations, and expansion of advanced therapy manufacturing hubs.
Asia-Pacific
High growth potential due to rising biopharma investment, expanding biosimilar production, and increasing adoption of modular facilities.
Latin America
Growing focus on vaccine production and biosimilar manufacturing creates new opportunities for next-generation technologies.
Middle East & Africa
Emerging investments in local pharmaceutical production and efforts to build regional biomanufacturing capacity.
End-User Industries
-
Biopharmaceutical companies
-
Contract development and manufacturing organizations (CDMOs)
-
Research institutes
-
Clinical research organizations
-
Academic laboratories
-
Vaccine manufacturers
Market Size and Factors Driving Growth
The global next-generation biomanufacturing market size was valued at USD 26.61 billion in 2024 and is expected to reach USD 56.43 billion by 2032, at a CAGR of 9.85% during the forecast period
Major drivers include technological innovation, increased global population, rising chronic disease prevalence, sustainability goals, and strong government support for biologics production. AI-driven bioprocess optimization improves predictive control, enhances yield, and lowers production costs. Single-use systems reduce water and energy consumption, supporting sustainability initiatives.
Opportunities are strong in emerging regions where governments seek to build national biologics manufacturing capacity. Investments in biosimilars, vaccines, and advanced therapies create new markets for flexible, modular, and automated manufacturing platforms.
Conclusion
The Next-Generation Biomanufacturing Market is positioned for strong growth as global demand for biologics accelerates. Advances in continuous bioprocessing, automation, and synthetic biology are transforming production infrastructure. Flexibility, efficiency, scalability, and sustainability are central themes shaping future developments.
Innovation in digital tools, smart sensors, and AI-driven systems will continue to drive process optimization and quality enhancement. Stakeholders have robust opportunities in areas such as modular facilities, personalized therapy manufacturing, and low-cost bioproduction for emerging markets.
The industry’s long-term success depends on resilient supply chains, skilled workforce development, and regulatory harmonization. Continued investment in next-generation platforms will enable broader global access to life-saving biologics.
Frequently Asked Questions (FAQ)
What is next-generation biomanufacturing?
Next-generation biomanufacturing refers to advanced systems that enhance efficiency, flexibility, and scalability in producing biologics using technologies such as continuous processing, single-use systems, automation, and synthetic biology.
Which technologies are driving the market?
Key technologies include continuous bioprocessing, single-use platforms, AI-driven control systems, digital twins, and synthetic biology.
What is the projected market size by 2035?
The market is expected to reach approximately USD 110 billion by 2035, driven by strong adoption of advanced manufacturing systems.
Which regions lead the market?
North America and Europe lead due to strong biopharma industries, while Asia-Pacific shows the fastest growth due to expanding manufacturing infrastructure.
What are the biggest challenges for next-generation biomanufacturing?
Challenges include regulatory complexity, high capital investment, supply chain limitations, and shortages of skilled professionals.
What industries use next-generation biomanufacturing?
Biopharmaceutical companies, CDMOs, vaccine developers, research institutions, and advanced therapy manufacturers rely heavily on next-generation technologies.
Browse More Reports:
Global Fracking Water Treatment Market
Global Giardiasis Treatment Market
Global High Purity Limestone Market
Global Luciferase Assay Kits Market
Global RF Over Fiber Market
Global Solar Photovoltaic (PV) Mounting Systems Market
Global Handheld Intraoral Dental 3D Scanners Market
Global Metalworking Fluid Additives Market
Global Pet Food Flavors Market
Global Polypropylene Compounds Market
Global Time Temperature Indicator Labels Market
Global Digital X-Ray Market
Global Insulin Delivery Devices Market
Global Acrylonitrile Butadiene Rubber (BR) Market
Global Aerogel Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com